Basic Photophysics
1.1. Kinetics of Luminescence
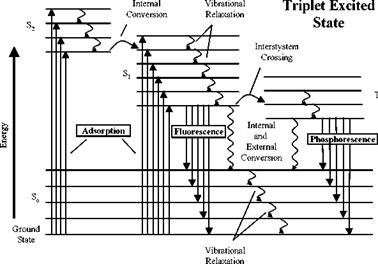
Pressure sensitive paint (PSP) and temperature sensitive paint (TSP) are, respectively, based on the oxygen and thermal quenching processes of luminescence which are reversible processes in molecular photoluminescence. The general principles of luminescence are described in detail by Rebek (1987), Becker (1969) and Parker (1968). The different energy levels and photophysical processes of luminescence for a simple luminophore can be clearly described by the Jablonski energy-level diagram shown in Fig. 2.1. The lowest horizontal line represents the ground-state energy of the molecule, which is normally a singlet state denoted by S0. The upper lines are energy levels for the vibrational states of excited electronic states. The successive excited singlet and triplet states are denoted by S1 and Sz and T1, respectively. As is normally the case, the energy of the first excited triplet state T1 is lower than the energy of the corresponding singlet state S1.
A photon of radiation is absorbed to excite the luminophore from the ground electronic state to excited electronic states (S0 ^ Sj and S0 ^ S2). The excitation process is symbolically expressed as S0 + hv ^ Sj, where h is the Plank constant and v is the frequency of the excitation light. Each electronic state has different vibrational states, and each vibrational state has different rotational states. The excited electron returns to the unexcited ground state by a combination of radiative and radiationless processes. Emission occurs through the radiative processes called luminescence. The radiation transition from the lowest excited singlet state to the ground state is called fluorescence, which is expressed as Sj ^ S0 + hvf. Fluorescence is a spin-allowed radiative transition between two states of the same multiplicity. The radiative transition from the triplet state to the ground state is called phosphorescence (Tj ^ S0 + hvp ), which is a spin-
forbidden radiative transition between two states of different multiplicity. The lowest excited triplet state, T1, is formed through a radiationless transition from S1 by intersystem crossing (Sj ^ Tj). Since phosphorescence is a forbidden transition, the phosphorescent lifetime is typically longer than the fluorescent lifetime. Luminescence is a general term for both fluorescence and phosphorescence.
|
Singlet Excited States
Fig. 2.1. Jablonsky energy-level diagram |
Radiationless deactivation processes mainly include internal conversion (IC), intersystem crossing (ISC) and external conversion (EC). The internal conversion (IC) is a spin-allowed radiationless transition between two states of the same multiplicity (S2 ^ Sj, Sj ^ S0). Typically, this process is expressed as Sj ^ S0 + A, where A denotes heat released. IC appears to be particularly efficient when two electronic energy levels are sufficiently close. The intersystem crossing (ISC) is a spin-forbidden radiationless transition between two states of the different multiplicity, which are expressed as Sj ^ Tj + A and Tj ^ S0 + A. Phosphorescence depends to a large extent on the population of the triplet state (Tj) from the excited singlet state (Sj) by the intersystem crossing. In addition, deactivation of an excited electronic state may involve interaction and energy transfer between the excited molecules and the environment like solutes, which are called external conversion (EC).
The excited singlet and triplet states can be deactivated by interaction of the excited molecules with the components of a system. These bimolecular processes are quenching processes, including collisional quenching (diffusion or nondiffusion controlled), concentration quenching, oxygen quenching, and energy transfer quenching. The oxygen quenching of luminescence is the major photophysical mechanism for PSP. Due to the oxygen quenching, air pressure on
an aerodynamic model surface is related to the luminescent intensity by the Stern – Volmer equation that will be further discussed. The quantum efficiency of luminescence in most molecules decreases with increasing temperature because the increased frequency of collisions at elevated temperatures improves the possibility for deactivation by the external conversion. This effect associated with temperature is the thermal quenching, which is the major photophysical mechanism for TSP.
The population of the excited singlet states (SI) and triplet states (TI) at any given time depends on the competition among different photophysical processes listed in Table 2.1. The singlet state population [SI] and triplet state population [TI] are described by the following first-order kinetic model
йЫ. = I a – (kf + kic + + kq(s)[Q])[Sj]
where Ia is the light absorption rate of generating the excited singlet states, [Q] is the population of the quencher Q, kf and kp are, respectively, the rate constants for fluorescence and phosphorescence, kisc(si } and kisc(ti } are, respectively, the rate constants for the intersystem crossings SI ^ TI and TI ^ S0, kcc is the rate constant for the internal conversion, and kq(s) and kq(t) are the rate constants for the quenching in the singlet states and triplet states, respectively. The light absorption rate Ia = ksI[S0] is proportional to the population [S0] in the ground state and the rate constant of excitation ks1. After a pulse excitation, the times required for the populations in the excited singlet state and triplet state to decay to 1/e of the initial value are, respectively,
Tf = (kf + kic + kisc(sI-tI) + kq(s)[Q])
Tp = I/(kp + kisc(ti-So) + kq(t) [Q]). (2.2)
The time constants Tf and Tp are defined as the fluorescent and phosphorescent
lifetimes, respectively. Usually, the lifetime of a specific photophysical process is defined as the reciprocal of the corresponding rate constant. Typical values of the lifetimes for different photophysical processes are listed in Table 2.1. When the intersystem crossing from T1 back to Sq (T1 ^ S1 + A ) is included in the kinetic
m°deb extra terms kisc(tl-Sl)[Tj] and – kisc(,I-Sl)[Ti] should be adde^
respectively, to the right-hand sides of Eq. (2.1) for [SI] and [TI], where ksx( ) is the rate constant for the intersystem crossing Tq ^ Sq. In this case,
the kinetic model becomes a coupled system of equations (Mosharov et al. 1997; Bell et al. 2001). Since S1 is a higher energy state than T:, this intersystem crossing is thermally activated and therefore the rate constant for the process T1 — S: is temperature-dependent.
|
Table 2.1. Photophysical processes involving electronically excited states
|