Time Response of Porous Pressure Sensitive Paint
8.1.2. Deviation from the Square-Law
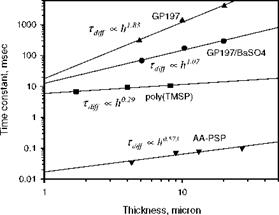
Compared to a conventional homogeneous PSP, a porous PSP has a much shorter diffusion time ranging from 18 |j. s to 500 |j. s due to enlarged air-polymer interface (Sakaue and Sullivan 2001; Sakaue et al. 2002a). Interestingly, recent measurements of the response time for three polymers, GP197, GP197/BaSO4 mixture and Poly(TMSP), show that the classical square-law estimate Eq. (8.13) does not hold for a porous PSP (Teduka 2001; Asai et al. 2001). As shown in Fig. 8.4, measurements gave the power-law relations for the diffusion timescale Tdiff <x h18 for GP197, Tdiff <x h107 for GP197/BaSO4 mixture, and Tdiff ^ h029 for Poly(TMSP) at 313.1 K. For a porous anodized aluminum (AA) surface, the power-law relation is Tdff h0573 (Sakaue 1999; Sakaue and Sullivan 2001). For
the GP197 silicone polymer, the power-law exponent is close to 2 as predicted by the classical estimate for a homogenous polymer film. However, the power-law exponent for the porous materials GP197/BaSO4 mixture, Poly(TMSP), and AA – PSP is significantly smaller than 2. In addition, Figure 8.5 shows that the power – law exponent for the polymer Poly(TMSP) linearly increases with temperature over a temperature range of 293.1-323.1 K. In order to understand the time response of a porous PSP, from a standpoint of phenomenology, Liu et al. (2001b) derived the expressions for the effective diffusivity and diffusion timescale of a porous layer.
|
Fig. 8.4. The power-law relationship between the response time and coating thickness for three polymers GP197, GP197/BaSO4 mixture and Poly(TMSP) at 313.1 K, and AA surface at about 300K. Experimental data are from Teduka (2001), Asai et al. (2001), and Sakaue(1999) |