Dimensions and Conversions
SI basic units and SI derived units are listed of the major flow, transport, and thermal quantities. In the left column name and symbol are given and in the right column the unit (dimension), with the symbol in ^ [ ] used in Appendix C, and in the line below its conversion to the U. S. customary units.
3
 2.75
2.75
2.5
2.25 2
1.75
d
1.5
1.25 1
0.75 0.5 0.25 0
0 5 10 15 20 25 30
kT/e
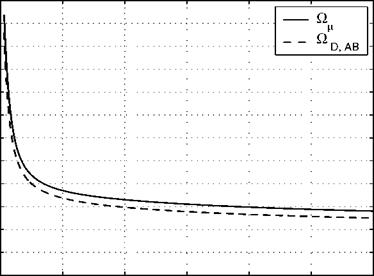
Fig. B.1. Dimensionless collision integrals = Ok, and ODAB of air as functions
of kT/є or kT/єав [4, 6].
|
SI Basic Units
amount of substance, mole |
[kg-mol], ^ [mole]
1 kg-mol = 2.20462 lbm-mol
|
SI Derived Units |
|
|
area, A |
[m2], ^ [L2] 1 m2 = 10.76391 ft2 |
|
volume, V |
[m3], ^ [L3] 1.0 m3 = 35.31467 ft3 |
|
speed, velocity, v, u |
[m/sL ^ [L/t] 1.0 m/s = 3.28084 ft/s |
|
force, F |
[N] = [kg m/s2], ^ [M L/t2] 1.0 N = 0.224809 lb/ |
|
pressure, p |
[Pa] = [N/m2], ^ [M/L t2] 1.0 Pa = 10~5 bar = 9.86923-10~6 atm = = 0.020885 lb//ft2 |
|
density, p |
[kg/m3], ^ [M/L3] 1.0 kg/m3 = 0.062428 lbm/ft3 |
|
(dynamic) viscosity, p |
[Pa s] = [N s/m2], ^ [M/L t] 1.0 Pa s = 0.020885 lb/ s/ft2 |
|
kinematic viscosity, v |
[m2/s], ^ [L2/t] 1.0 m2/s = 10.76391 ft2/s |
|
shear stress, т |
[Pa] = [N/m2], ^ [M/L t2] 1.0 Pa = 0.020885 lb/ /ft2 |
|
energy, enthalpy, work, quantity of heat |
[J] = [N m], ^ [M L2/t2] 1.0 J = 9.47813-10-4 BTU = = 23.73036 lbmft2/s2 = 0.737562 lb//s2 |
(mass specific) internal energy, [J/kg] = [m2/s2], ^ [L2/t2] enthalpy, e, h 1.0 m2/s2 = 10.76391 ft2/s2
|
(mass) specific heat, cv, cp specific gas constant, R |
[J/kg K] = [m2/s2 K], ^ [L2/t2T] 1.0 m2/s2K = 5.97995 ft2/s2 °R |
|
power, work per unit time |
[W] = [J/s] = [N m/s], ^ [M L2/t3] 1.0 W = 9.47813-10-4 BTU/s = = 23.73036 lbmft2/s3 |
|
thermal conductivity, к |
[W/m K] = [N/s K], ^ [M L/t3T] 1.0 W/m K = 1.60496-10-4 BTU/s ft °R |
= 4.018342 lbm ft/s3 °R
heat flux, q [W/m2] = [J/m2s], ^ [M/t3]
1.0 W/m2 = 0.88055-10-4 BTU/s ft2 = = 2.204623 lbm/s3
(binary) mass diffusivity, Dab [m2/s], ^ [L2/t]
1.0 m2/s = 10.76391 ft2/s
thermo diffusivity, D’A [kg/m s], ^ [M/L t]
1.0 kg/m s = 0.67197 lbm/ft s
diffusion mass flux, j [kg/m2s], ^ [M/L2t]
1.0 kg/m2s = 0.20482 lbm/ft2s
References
1. Taylor, B. N. (ed.): The International System of Units (SI). US Dept. of Commerce, National Institute of Standards and Technology, NIST Special Publication 330, 2001, US Government Printing Office, Washington, D. C. (2001)
2. Taylor, B. N. (ed.): Guide for the Use of the International System of Units (SI). US Dept. of Commerce, National Institute of Standards and Technology, NIST Special Publication 811, 1995, US Government Printing Office, Washington, D. C. (1995)
3. Hirschfelder, J. O., Curtiss, C. F., Bird, R. B.: Molecular Theory of Gases and Liquids. John Wiley & Sons, New York (1966)
4. Bird, R. B., Stewart, W. E., Lightfoot, E. N.: Transport Phenomena, 2nd edn. John Wiley & Sons, New York (2002)
5. Vincenti, W. G., Kruger, C. H.: Introduction to Physical Gas Dynamics. John Wiley & Sons, New York (1965), Reprint edition, Krieger Publishing Comp., Melbourne, Fl (1975)
6. Neufeld, P. D., Jansen, A. R., Aziz, R. A.: J. Chem. Phys. 57, 1100-1102 (1972)












