Computation Models
The relations for the determination of the transport properties viscosity p and thermal conductivity k, which we have considered in the preceding subsections, are valid in the lower temperature domain, i. e., as long as dissociation does not occur. Air begins to dissociate at temperatures between 1,500 K and 2,000 K, depending on the density level. In general the following holds: the lower the density, the lower is the temperature at which dissociation occurs.
If appreciable dissociation is present, the transport properties are determined separately for each involved species. Mixing formulas (exact, and approximate ones such as Wilke’s and other [2, 3]) are then employed in order to determine the transport properties of dissociated air.
Wilke’s semi-empirical formula, for example, reads [2]:
with
where pi, xi and Mi are viscosity, mole fraction and molecular weight of the species i, respectively, and j is a dummy subscript.
Wilke’s formula is used in aerothermodynamics with good results, also for the determination of the thermal conductivity of gas mixtures. For multicomponent diffusion coefficients the available mixing formulae, [2], are less satisfactory [9].
The transport properties of gas mixtures in thermo-chemical equilibrium are always functions of two thermodynamic variables, for example internal energy e and density p.
The nowadays fully accepted theoretical base for the determination of transport properties is the Chapman-Enskog theory for gases at “low” density with extensions also for dissociated air in equilibrium or in non-equilibrium. In the methods of numerical aerothermodynamics curve-fitted state surfaces are employed, see, e. g., [10], in order to obtain in a fast and exact manner the needed data. They use extensive data bases, e. g., [11]—[13].
In general it is accepted that transport properties of multi-species air at the flow conditions considered here can be determined to a sufficient degree of accuracy [9, 14]. The situation is different with flow problems exhibiting very high temperatures, and with combustion and combustion-wake problems of hypersonic flight propulsion systems.
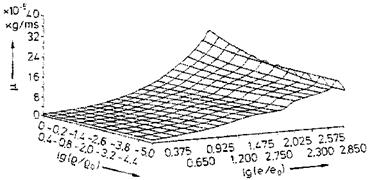
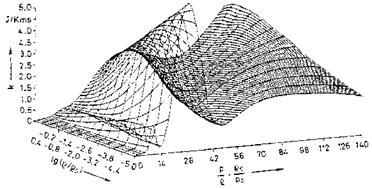
Examples of curve-fitted state surfaces of viscosity p(p, e) and thermal conductivity k(p, p/p) [10] for the typical pressure/density/internal energy range encountered by the flight vehicle classes in the background of this book are shown in Figs. 4.6 and 4.7.[31]
|
Fig. 4.6. Viscosity p of air as function of the density p and the internal energy e [10]. Database: [11] (p0 = 1.243 kg/m3, eo = 78,408.4 m2/s2). |
|
Fig. 4.7. Thermal conductivity k of air as function of the density p and the ratio pressure/density p/p [10]. Database: [11] (p0 = 1.292 kg/m3, p0 = 1.0133-106 Pa). |