Cryogenic Paints
A challenging problem is application of PSP in cryogenic wind tunnels like the NASA Langley National Transonic Facility (NTF) and European Transonic Wind Tunnel Facility (ETW). Porous materials were usually used as binders for PSP at cryogenic temperatures. Porous material has a large exposure surface area where the probe luminophore can be directly applied and luminescence can be directly quenched by oxygen. The use of porous materials as binders allows PSP measurements at cryogenic temperatures and achieves fast time response as well (in the order of microseconds). Various porous materials were investigated as binders for PSP, including a thin layer chromatography (TLC) plate (Baron et al. 1993), hydrothermal coating (Bacsa and Gratzel 1996), sol-gel (MacCraith et al. 1995; Jordan et al. 1999a, 1999b), tape-casting (Scroggin 1999), anodized aluminum (Asai 1997a), anodized titanium and porous paper filter (Erausquin 1998; Erauquin et al. 1998).
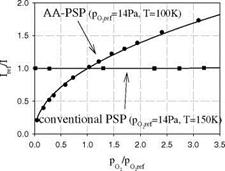
Aluminum can be anodized to create a thin aluminum oxide layer on the surface through an electrochemical process. Anodized aluminum (AA) is highly porous with 10-100 nm micropores uniformly distributed on the surface. AA-PSP is made by adsorbing the luminophore into the pores on the AA surface (Asai 1997a). Figure 3.19 shows the Stern-Volmer plot for an AA-PSP, Ru(dpp) on anodized aluminum, compared with a conventional polymer-based PSP (Ru(dpp) in RTV) at cryogenic temperatures. This AA-PSP still exhibits good sensitivity to oxygen even at 100 K, whereas the conventional PSP loses its sensitivity to oxygen at 150 K. Upchurch et al. (1998) and Asai et al. (2000, 2002) developed a polymer-based cryogenic PSP that used highly porous Poly(TMSP) as a binder.
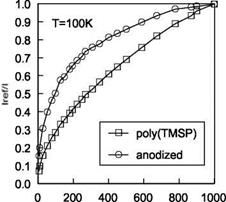
To compare the two cryogenic PSP formulations, Figure 3.20 shows the Stern – Volmer plots for the Poly(TMSP)-based PSP and AA-PSP that use Ru(dpp) as a luminescent dye; both PSPs exhibit the non-linear behavior in the Stern-Volmer plot.
|
Fig. 3.19. The Stern-Volmer plots for a porous AA-PSP and a conventional PSP with silicone rubber as a binder at cryogenic temperatures. Both PSP formulations uses Ru(ph2- phen) as a probe luminophore. From Sakaue (1999) |
|
oxygen concentration, ppm Fig. 3.20. Comparison of Poly(TMSP) PSP with anodized aluminum (AA) PSP at 100 K, where excitation is at 400+50 nm and emission is at 650+50 nm. From Asai et al. (2000) |
A family of the luminescent molecules Ru(trpy) (see Fig. 3.21 for the chemical structure) has been studied for making cryogenic TSP formulations since it is found that these compounds are temperature sensitive at cryogenic temperatures (Campbell 1994; Erausquin 1998; Iijima et al. 2003). They have very intense emission at low temperatures, but they are nearly fully quenched at the room temperature. The absorption and emission spectra of the family of Ru(trpy) are
very similar to those of Ru(dpp) and Ru(bpy). The Ru(trpy) compounds, which can be excited by either a UV light or blue light, emit red luminescence. Various [Ru(trpy)2] molecules with different ligands were synthesized (Erausquin 1998). The dynamics of the metal-to-ligand bond, as well as the electron donating or accepting characteristics of the ligand, has a significant effect on the temperature sensitivity of a luminescent molecule. Thus, this would enable synthesis of a molecule specifically designed to respond with high sensitivity over a certain range of cryogenic temperatures.
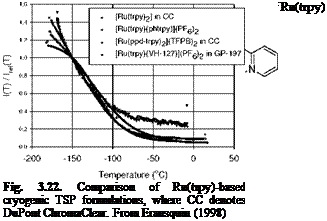
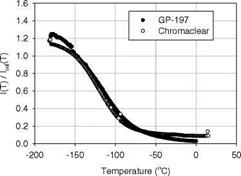
DuPont ChromaClear (CC) is selected as a binder for cryogenic TSP due to its low oxygen diffusivity and good surface adhesion at cryogenic temperatures (Erausquin 1998). Figure 3.22 shows a comparison of several synthesized [Ru(trpy)2] compounds in the polymers CC and GP-197. In general, interaction between a polymer binder and a luminescent molecule can affect the mobility of the metal-to-ligand bonds, changing the temperature dependency of the paint. Figure 3.23 shows the temperature dependencies for [Ru(trpy)(phtrpy)](PF6)2 in two different polymer binders CC and GP-197. Other Ruthenium compounds have a good response at cryogenic temperatures as well, as shown in Table 3.3 that summarizes the temperature sensitivities and useful temperature ranges for cryogenic TSP formulations calibrated by Erausquin (1998).
|
Fig. 3.23. Temperature calibration for cryogenic TSPs: [Ru(trpy)(phtrpy)](PF6)2 in GP-197 and DuPont ChromaClear. From Erausquin (1998) |
|
Table 3.3. Cryogenic temperature sensitive paints
|