Air pressure density and temperature
Air molecules are always in a state of rapid random motion. When they strike a surface, they bounce off, and in doing so, produce a force, just as you could
|
Fig. 1.9 Thick cambered section at the wing root of this piston-engined Hermes airliner of the early postwar period (Photographed at Duxford museum) |
produce a force on a wall by throwing handfuls of pebbles against it. We describe the magnitude of pressure in terms of the force that the molecular impacts would produce per square metre (or square foot) of surface.
The air density (p) is the mass (quantity) of air in each cubic metre and the density therefore depends on how many air molecules are contained in that volume. If we increase the number of molecules in a given volume without altering their rate of movement, the force due to pressure will increase, since there will be more impacts per square metre.
The rate at which air molecules move is determined by the temperature. Raising the temperature increases the rate of molecular movement, and hence tends to increase the pressure.
It will be seen, therefore, that the air pressure is related to its density and temperature. Students of engineering may care to note that the relationship is given by the gas law p = pRT, where R is a constant.
The pressure, temperature and density of the atmospheric air all reduce significantly with increasing altitude. The variation is described more fully in Chapter 7. The reduction in density is a particularly important factor in aircraft flight, since aerodynamic forces such as lift and drag are directly related to the air density.
|
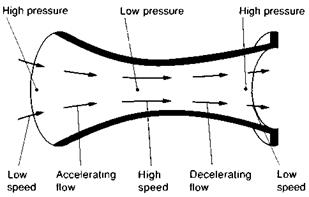
Fig. 1.10 Pressure and speed The air accelerates when flowing from a high pressure to a low one, and slows down when flowing from a low pressure to a high one |