First Law of Thermodynamics
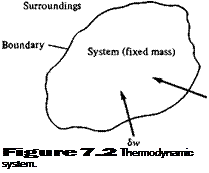 |
Consider a fixed mass of gas, which we define as the system. (For simplicity, assume a unit mass, for example, 1 kg or 1 slug.) The region outside the system is called the surroundings. The interface between the system and its surroundings is called the boundary, as shown in Figure 7.2. Assume that the system is stationary. Let Sq be an incremental amount of heat added to the system across the boundary, as sketched in Figure 7.2. Examples of the source of 8q are radiation from the surroundings which is absorbed by the mass in the system and thermal conduction due to temperature gradients across the boundary. Also, let 8 w denote the work done on the system by the surroundings (say, by a displacement of the boundary, squeezing the volume of the system to a smaller value). As discussed earlier, due to the molecular motion of the gas, the system has an internal energy e. The heat added and work done on the system cause a change in energy, and since the system is stationary, this change in
Sq + Sw = de
![]() This is the first law of thermodynamics: It is an empirical result confirmed by experience. In Equation (7.11), e is a state variable. Hence, de is an exact differential, and its value depends only on the initial and final states of the system. In contrast, Sq and Sw depend on the process in going from the initial to the final states.
This is the first law of thermodynamics: It is an empirical result confirmed by experience. In Equation (7.11), e is a state variable. Hence, de is an exact differential, and its value depends only on the initial and final states of the system. In contrast, Sq and Sw depend on the process in going from the initial to the final states.
For a given de, there are in general an infinite number of different ways (processes) by which heat can be added and work done on the system. We are primarily concerned with three types of processes:
1. Adiabatic process. One in which no heat is added to or taken away from the system
2. Reversible process. One in which no dissipative phenomena occur, that is, where the effects of viscosity, thermal conductivity, and mass diffusion are absent
3. Isentropic process. One that is both adiabatic and reversible
For a reversible process, it can be easily shown that Sw = —pdv, where dv is an incremental change in the volume due to a displacement of the boundary of the system. Thus, Equation (7.11) becomes
![]() Sq — p dv = de
Sq — p dv = de













