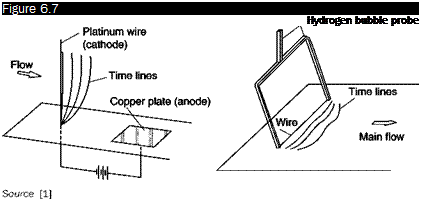
Hydrogen bubbles
If a difference of potential is applied between a metallic wire immersed in a stream of water and a metal plate on the bottom of the channel, by
|
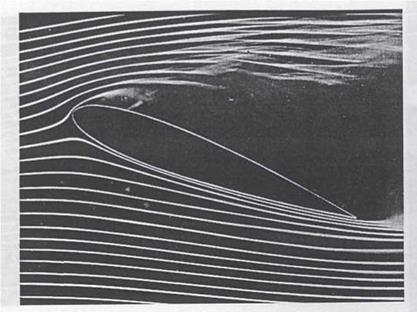
Stall of an airfoil visualized with smoke filaments
|
electrolysis of water from the wire, a continuous stream of bubbles of hydrogen or oxygen is generated, depending on the sign of the difference of potential applied. It is preferred that the wire is the cathode since the amount of bubbles of hydrogen produced is twice that of oxygen. Wires with a diameter of the order of hundredths of millimeters are used so that the bubbles, which have a size comparable to the diameter of the wire, do not rise too quickly to the surface. Adding an electrolyte to water is not strictly needed because the usual presence of salts dissolved in tap water is sufficient; anyway sodium chloride or sodium sulfate can be added to water. The applied voltages are 100-200 V.
If the cathode is a thin wire normal to the direction of the stream and a short electrical pulse is applied, a line of hydrogen bubbles is generated along the wire (Figure 6.7).
With a power supply pulsed with a constant frequency, successive lines of bubbles are obtained separated by a constant time interval (time lines). These lines are carried by the stream and deform according to the profiles of local speed.
Also, if the wire is partially covered with an insulating varnish, emission of bubble packs can be achieved which provide an indication of the speed profiles in the stream (Figure 6.8). The time period during which the
 |
Time lines generated with hydrogen bubbles
bubbles survive in the stream is limited. The spread of bubbles increases with the Reynolds number and is very rapid in turbulent flow. The application of the method is therefore limited to very slow streams, with speeds of the order of 20-30 cms-1.