Molecular Origin of the Thermodynamic Pressure
As an example of the molecular basis of important properties, we first examine the origin of the thermodyamic pressure. Consider a uniform gas contained in a box.
|
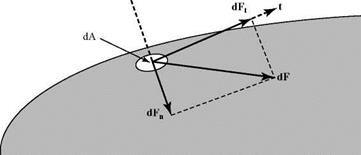
Figure 2.2. Resolution of surface forces into parallel and normal components. |
From kinetic theory, we know that the pressure is the reaction force generated by the interactions of the molecules with the box surfaces. Pressure is defined as the force per unit area arising from these collisions. Because the interaction is (in the first approximation) an elastic collisions, then the net reaction force vector is perpendicular to the surface at any point. If one-third of the molecules are traveling in each of three mutually perpendicular directions (e. g., axes parallel to the edges of the box) at a given time, and half of those going in any particular direction are traveling away from the surface on average, then one-sixth of the molecules in a layer of thickness dx strikes the surface in a time dt = dx/c, where c is the average velocity, as already defined. If m is the mass of each molecule and the momentum is reversed in the elastic collision with the surface, then the reaction force in each collision is dF = 2mc. Thus, if n is the number of molecules per unit volume, the pressure is:
![]() (2.3)
(2.3)
because p = nm is the density, or mass per unit volume. This shows that the pressure is proportional to the kinetic energy per unit mass due to random molecular motion.