Typical Pressure Sensitive Paints
A typical PSP is prepared by dissolving a luminescent dye and a polymer binder in a solvent; the order of mixing the components and the relative concentration of the components may change the characteristics of the paint. Chlorinated organic solvents such as dichloromethane and trichloroethane have been used for making PSP. The selection of a polymer binder for PSP is important, which should be based on a balanced consideration of its oxygen permeability, temperature effect, humidity effect, adhesion, mechanical stability, photodegradation, and other required properties. Silicone rubbers, GP-197, silica gel and sol-gel-derived coatings have been used as binders for PSPs and oxygen sensors (Wan 1993; Gallery 1993; Xu et al. 1994; MacCraith et al. 1995; Jordan et al. 1999a, 1999b). Other polymers and porous materials that are potentially useful for PSP can be found in the literature of polymers (Krevelen 1976; Mulder 1991; Fried 1995; Robinson and Perlmutter 1994). The permeability, solubility and diffusion coefficients of a polymer binder are related to the pressure sensitivity and time response of PSP. Furthermore, the behavior of PSP depends on interaction between a probe molecule and its surrounding polymer. The microenvironment of the probe molecule in the polymer binder can significantly affect the luminescence and quenching behavior (Hartmann et al. 1995; Meier et al. 1995; Xu et al. 1994; Lu and Winnik 2001; Lu et al. 2001). Also, it was observed that a basecoat might affect the behavior of PSP (Coyle et al. 1995). A useful review on quenching of luminescence by oxygen in polymer films was given by Lu and Winnik (2000), stressing on luminescent materials and polymers.
Table 3.1 lists some PSP formulations along with their spectroscopic properties and the Stern-Volmer coefficients. In Table 3.1, the Stern-Volmer coefficients A(T) and B(T) are the coefficients in the linear relation
Iref/1 = A(T) + B(T)p/pref at the room temperature of about 20oC, where the reference pressure pref is the ambient pressure of 1 atm. For certain PSPs that do not completely obey the linear Stern-Volmer relation, the coefficients A(T) and B( T ) are estimated by fitting data over a finite linear range. The results are collected from the theses and papers by Wan (1993), Burns (1995), Baron et al.
(1993) , Kavandi et al. (1990), and McLachlan et al. (1993a, 1995), which documented the absorption spectra, emission spectra, and Stern-Volmer plots of oxygen-sensing luminophores and supporting polymer matrices. Table 3.1 also include a number of proprietary PSPs developed by TsAGI (Troyanovsky et al. 1993; Bukov et al. 1993), the former McDonnell Douglas (now Boeing at St. Louis) (Morris et al. 1993a, 1993b; Morris 1995), the University of Washington/NASA Ames (McLachlan and Bell 1995) and NASA Langley (Oglesby and Jordan 2000). Some PSP formulations of the former McDonnell Douglas have been patented (Schwab and Levy 1994). Generally speaking, a good PSP has the Stern-Volmer coefficient B(T) larger than 0.5, indicating acceptable pressure sensitivity for quantitative measurements (Oglesby et al. 1995a).
|
Table 3.1. Pressure sensitive paints
|
Three families of luminescent dyes, Platinum Porphyrins, Ruthenium Polypyridyls and Pyrene derivatives, have been commonly used for making PSP. Recipes of three PSP formulations are given in Appendix B. The Platinum Porphyrin compounds, which can be excited by either an UV light or a green light, emit red luminescence. They are very sensitive to oxygen, but they often have a long lifetime and low luminescent intensity at the atmospheric pressure. The Ruthenium compounds also emit red luminescence when excited by either an UV light or a blue light. They are very photo-stable, but are difficult to incorporate into polymer systems. The Pyrene derivatives, which are UV excited, emit blue
luminescence. The Pyrene derivatives have weak temperature sensitivity; however, they suffer from photodegradation and sublimation.
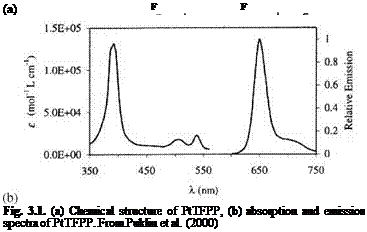
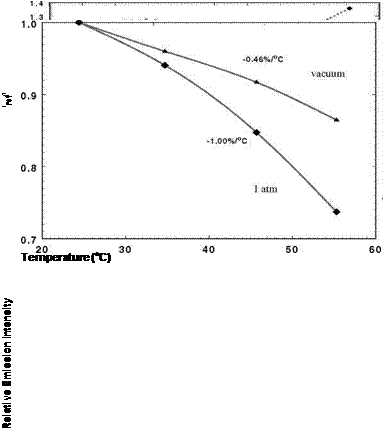
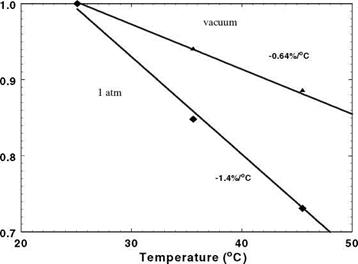
Figure 3.1 shows the chemical structure, and absorption and emission spectra for platinum meso-tetra(pentafluorophenyl)porphine (PtTFPP). Figures 3.2 and 3.3 show the Stern-Volmer plots and temperature dependencies for two PtTFPP PSP formulations: PtTFPP in the FIB polymer (poly-heptafluoro-n-butyl methacrylate-co-hexafluorisopropyl methacrylate) developed by the University of Washington (Gouterman and Carlson 1999) and PtTFPP in the FEM polymer (poly-tifluoro-ethylmethacrylate-co-isobutylmethacrylate) developed by NASA Langley (Oglesby and Jordan 2000). In these figures, the lower temperature sensitivity of PSP at vacuum represents the intrinsic temperature dependency of the luminophore, while the higher temperature sensitivity of PSP at the atmospheric pressure indicates an additional temperature effect on the oxygen diffusion in the polymer.
 |
(b)
Fig. 3.2. (a) The Stern-Volmer plots, and (b) temperature dependency for PtTFPP in the FIB Polymer. From Oglesby and Jordan (2000)
![]()
|
|
|
|
|
|
 |
|
|
||
|
||
|
||
In order to examine the effect of a polymer binder on the properties of PSP, Mebarki and Le Sant (2001) calibrated five PSP formulations that used the same porphyrin molecule, PtTFPP, with different polymer binders. Two formulations, the PAR PSP from the Institute for Aerospace Research (IAR) of NRC in Canada (Mebarki 2000) and the FEM PSP from NASA Langley (Oglesby and Upchurch 1999), are not commercially available. Other paints, the FIB PSP originally developed by the University of Washington (Gouterman and Carlson 1999), sol – gel PSP (Jordan et al. 1999a, 1999b) and the Uni-Coat PSP (Mebarki 2000), were commercially produced by Innovative Scientific Solutions Inc. (ISSI). Except for the Uni-Coat PSP that did not require a primer layer, the commercial FIB and sol – gel PSP formulations were supplied with their respective primers. To simplify the application procedures and solve adhesion problems, the FIB active layer was applied on the top of the Tristar (DHMS C4.01TY3) white epoxy primer that was also used as a screen layer for both the FEM PSP and PAR PSP. It was found that the primer had no effect on the pressure or temperature sensitivity of the active layer. However, the polymer binder (or permeable matrix) in which the porphyrin molecule was immobilized affected both the pressure and temperature sensitivities of the paint. To evaluate the performance of PSP, the pressure sensitivity and temperature sensitivity are defined as SP = A(lref/l)/AP (in % per bar) and
ST = – A( I / lref )/ AT (in % per degree), respectively. The pressure sensitivity was calculated in a pressure range of 0.15-2 bars and the temperature sensitivity was determined in a temperature range of 10-35°C. Figure 3.4 shows the pressure sensitivity SP as a function of temperature and the temperature sensitivity ST as a function of pressure. The pressure sensitivity SP varied from 55% to nearly
80% per bar, depending on the polymer binder used and temperature as well. The FIB PSP formulation had nearly constant pressure sensitivity over a temperature range of 10-40°C. The Uni-Coat and sol-gel PSP formulations had a similar linear dependency of the pressure sensitivity SP on temperature; the temperature sensitivity ST ranges from 0.6% to 1.6% per degree. The temperature sensitivity was somewhat affected by pressure for all the PSP formulations except the FIB PSP. The FIB PSP also has the lowest temperature sensitivity among them.
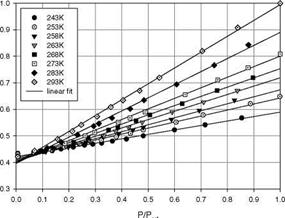
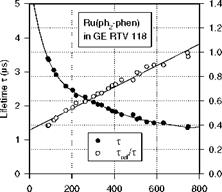
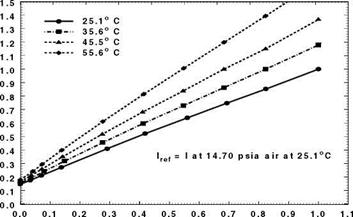
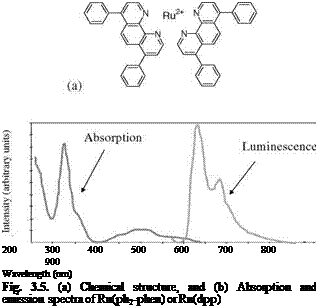
Figure 3.5 shows the chemical structure, and absorption and emission spectra of Bathophen Ruthenium Chloride (Ru(ph2-phen) or Ru(dpp)). Ruthenium-based oxygen sensors have been studied extensively by analytical chemists (Bacon and Demas 1987; Carraway et al. 1991a, 1991b; Sacksteder et al. 1993; Xu et al. 1994; Klimant and Wolfbeis 1995). The Ruthenium-based PSP formulations have been developed and used for wind tunnel testing by the former McDonnell Douglas (now Boeing at St. Louis) (Schwab and Levy 1994). Figure 3.6 shows the Stern – Volmer plots for Ru(dpp) in GE RTV 118 added with silica gel particles at different temperatures; Figure 3.7 shows the luminescent lifetime as a function of pressure for Ru(dpp) in GE RTV 118 at 22oC.
|
|
(b)
|
Fig. 3.6. The Stern-Volmer plots for Ru(ph2-phen) or Ru(dpp) in GE RTV 110 added with silica gel particles, where the reference pressure prf is 14.5 psi and reference temperature is 293 K. From Lachendro (2000) |
|
P (mmHg) Fig. 3.7. Lifetime-pressure relation for Ru(ph2-phen) in GE RTV 118 at 22oC, where Trf is the lifetime at the ambient pressure (1 atm). From Liu et al. (1997b) |
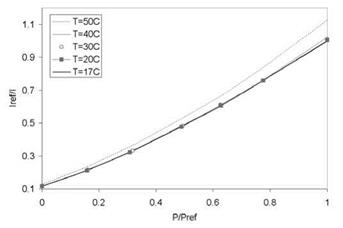
Figure 3.8 shows the chemical structure and absorption and emission spectra of Pyrene. The Pyrene-based PSP formulations were developed by TsAGI/OPTROD in Russia (Fonov et al. 1998). One of them was the binary paint (B1 PSP) in which a pressure-insensitive reference component was added to correct the excitation light variations on a surface in performing a ratio between the wind-on and wind-off images. Figure 3.9 shows the Stern-Volmer plots at different temperatures for Pyrene complex in GE RTV 118. Obviously, this Pyrene-based
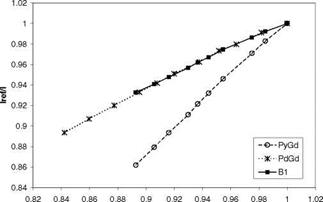
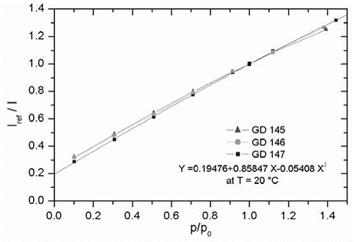
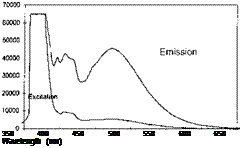
PSP exhibits weak temperature dependency over a temperature range of 17-40oC. Note that Perylene and its derivatives like Green Gold (perylene dibutylate) were also used as a luminescent dye for PSP. Besides TsAGI, ONERA in France and DLR in Germany developed Pyrene-based PSP formulations as well (Engler et al. 2001a). The PyGd PSP formulation developed by ONERA contained Pyrene as a pressure-sensitive dye and a gadolinium oxysulfide as a reference component. Figure 3.10 shows the emission spectrum of the PyGd PSP excited at 325 nm. The two components in the paint absorbed an ultraviolet excitation light and emitted at sufficiently different wavelengths such that the emissions from the two components can be separated using optical filters. Figure 3.11 shows the Stern – Volmer plots at the ambient temperature for three Pyrene-based PSP formulations: PyGd, B1 and PdGd. Because the temperature sensitivity of the reference component was similar to that of the Pyrene dye in the PyGd PSP, the temperature effect can be compensated by taking a ratio between the luminescent intensities from the pressure-sensitive component (Pyrene) and reference component. As a result, the PyGd PSP displayed very low temperature sensitivity of 0.05%/K. A number of ‘Gottingen Dyes’ (GD) were developed by DLR and the University of Gottingen, and three stable Pyrene-based paints, GD145, GD146 and GD147, were tested in wind tunnels (Engler and Klein 1997b). Figure 3.12 shows the pressure sensitivities of the Gottingen PSP formulations. A shortcoming of Pyrene-based paints is that sublimation may occur when temperature is greater than 40oC.
![]()
 |
(a)
(b)
Fig. 3.8. (a) Chemical structure of Pyrene, and (b) absorption and emission spectra of Pyrene. From Mebarki (2001)
 |
|
|
|
|
|
|
|